The combination of HCOOCH CH2 H2O, a seemingly simple grouping of formic acid (HCOOH), methylene (CH2), and water (H2O), holds immense significance in the world of organic chemistry. Despite being a subject of frequent misconceptions, this compound plays a vital role in various industries ranging from renewable energy to environmental science. In this article, we will decode the real chemistry behind HCOOCH CH2 H2O and explore its components in detail. We will also delve into its industrial applications, reaction mechanisms, and safety considerations, offering you a complete understanding of this fascinating topic.
Whether you are a student, an industrial chemist, or a sustainability advocate, this guide will help you grasp the complexity and practical utility of HCOOCH CH2 H2O.
Understanding the Components of HCOOCH CH2 H2O
To fully comprehend the significance of HCOOCH CH2 H2O, we must first understand the role of each of its components: formic acid, methylene, and water. These three molecules interact in several ways to drive chemical reactions that have wide-reaching applications in various industries.
Formic Acid (HCOOH)
Formic acid (HCOOH), the simplest carboxylic acid, is a highly reactive organic compound.
Properties:
- Molecular weight: 46.03 g/mol
- Boiling point: 100.8°C
- Density: 1.22 g/cm³
Uses:
Formic acid has several industrial applications:
- Reducing Agent: It is used in chemical processes to donate electrons, helping to reduce other substances.
- Leather and Textile Industries: It is involved in dyeing processes and fixing dyes onto materials.
- Agriculture: Used as a preservative in animal feed and for pest control.
Methylene (CH2)
Methylene (CH2) is a highly reactive intermediate that plays a key role in organic chemistry.
Role:
- Methylene is often part of larger molecules, especially in polymer synthesis, where it can act as a monomer or react with other compounds to form long-chain polymers.
- Properties:
- Molecular weight: 14.03 g/mol
- Boiling point: -92.2°C
- Density: N/A (Due to its instability as a free radical)
Methylene is critical in organic reactions, particularly in reactions that involve radicals or carbenes.
Water (H2O)
Water (H2O) is the universal solvent, essential for facilitating countless chemical reactions, including the hydrolysis of methyl formate.
Role in Reactions:
- Solvent: Water is the medium in which many chemical reactions take place, enabling the transfer of protons and facilitating various types of bond cleavage and formation.
- Properties:
- Molecular weight: 18.02 g/mol
- Boiling point: 100°C
- Density: 1.00 g/cm³
Water plays a crucial role in the hydrolysis of esters, such as methyl formate, and in maintaining the reaction equilibrium.
Properties Comparison
| Property | Formic Acid (HCOOH) | Methylene (CH2) | Water (H2O) |
|---|---|---|---|
| Molecular Weight | 46.03 g/mol | 14.03 g/mol | 18.02 g/mol |
| Boiling Point | 100.8°C | -92.2°C | 100°C |
| Density | 1.22 g/cm³ | N/A | 1.00 g/cm³ |
| Uses | Reducing agent | Reactive intermediate | Solvent |
The Hydrolysis of Methyl Formate
The key reaction involving HCOOCH CH2 H2O is the hydrolysis of methyl formate (HCOOCH₃), a common ester. This reaction exemplifies the interaction of formic acid, methylene, and water.
The Reaction
The hydrolysis of methyl formate with water leads to the formation of formic acid and methanol:
HCOOCH3+H2O→HCOOH+CH3OH\text{HCOOCH}_3 + \text{H}_2\text{O} \rightarrow \text{HCOOH} + \text{CH}_3\text{OH}HCOOCH3+H2O→HCOOH+CH3OH
This process is a nucleophilic substitution reaction, where water acts as the nucleophile. The steps in the reaction are as follows:
- Protonation of Ester: The ester’s carbonyl carbon becomes more electrophilic under acidic conditions, making it more susceptible to attack.
- Nucleophilic Attack by Water: The water molecule attacks the electrophilic carbonyl carbon, forming an intermediate.
- Bond Cleavage: The bond between the methyl group and the ester breaks, releasing methanol and protonated formic acid.
- Deprotonation: The protonated formic acid is neutralized to yield formic acid.
Factors Influencing Hydrolysis
Several factors influence the rate and efficiency of this hydrolysis reaction:
| Factor | Description | Effect |
|---|---|---|
| Catalysts | Acidic or basic catalysts can speed up the reaction | Accelerates the reaction by shifting the equilibrium |
| Temperature | Higher temperature increases kinetic energy | Enhances reaction rate |
| Water Availability | Excess water can drive the reaction forward | Favors hydrolysis |
| Reaction Environment | Acidic or basic pH levels | Influences the reaction pathway |
Understanding these factors is crucial for optimizing the hydrolysis process in both industrial and laboratory settings.
Industrial and Lab Applications of HCOOCH CH2 H2O
The chemistry of HCOOCH CH2 H2O is utilized in a variety of applications, especially in industries focused on energy, material science, and manufacturing.
Practical Applications
- Textile Processing: Formic acid is used to fix dyes to fabrics, ensuring long-lasting colors.
- Rubber Production: Formic acid helps coagulate latex, which is crucial in natural rubber production.
- Formic Acid Fuel Cells: Formic acid is being explored as an energy carrier in next-generation fuel cells, offering a potential alternative to hydrogen-based fuel cells.
- pH-Controlled Reactions: Formic acid is often used to regulate acidity in organic syntheses, providing tight control over reaction conditions.
- Polymer Synthesis: Methylene intermediates play a critical role in enhancing the properties of polymers such as elasticity and strength.
- Catalyst Screening: The reaction system is used to study various hydrogenation processes.
Laboratory Protocols
- Catalyst Optimization: Controlled catalysts are used to stabilize reactions and improve yields.
- Polymer Growth: Methylene-based monomers are used in polymerization reactions to create high-performance materials.
- Resin Synthesis: Methylene intermediates are essential for the efficient synthesis of resins.
Safety and Environmental Considerations
When working with HCOOCH CH2 H2O, it is crucial to adhere to proper safety protocols to prevent harm and minimize environmental impact.
Safety Precautions
| Chemical | Hazard | Precaution |
|---|---|---|
| Methyl Formate | Irritant | Use in ventilated areas |
| Formic Acid | Corrosive | Wear gloves and goggles |
| Methanol | Toxic | Avoid inhalation and skin contact |
Environmental Impact
- Effluent Treatment: Acidic waste should be neutralized to reduce toxicity before disposal.
- Water Recycling: Sustainable water management practices should be implemented to minimize resource usage.
Addressing Misconceptions About HCOOCH CH2 H2O
There are several misconceptions about HCOOCH CH2 H2O that can lead to confusion.
- Stable Compound Misunderstanding: HCOOCH CH2 H2O is not a single molecule but rather a reactive system of different compounds.
- Oversimplified Roles: Each component (formic acid, methylene, and water) plays a distinct and essential role in chemical reactions.
- Narrow Perception of Applications: While often perceived as useful only in basic organic synthesis, the applications of this system extend into areas like green energy and material science.
- Underestimated Safety Risks: The components of this system have real hazards and require careful handling to ensure safety.
Why HCOOCH CH2 H2O is Trending
The chemistry of HCOOCH CH2 H2O is gaining popularity because of its versatility and wide-reaching potential. From industrial manufacturing to green energy applications, understanding its role is becoming increasingly important in both theoretical and practical contexts.
Conclusion
The system represented by HCOOCH CH2 H2O, though often misunderstood, is a cornerstone in both organic chemistry and industrial applications. By understanding its components—formic acid, methylene, and water—you can appreciate the breadth of its utility and its importance in chemical reactions. Whether you’re involved in polymer synthesis, energy research, or environmental science, mastering this chemistry can open up new avenues for innovation and sustainable practices.
FAQs
1. Is HCOOCH CH2 H2O a real chemical compound?
No, HCOOCH CH2 H2O is not a single chemical compound. It represents a system involving formic acid, methylene, and water in chemical reactions.
2. What are the common uses of HCOOCH CH2 H2O?
While HCOOCH CH2 H2O is not a specific compound, its components are widely used in chemical synthesis, fuel cell technology, and material science.
3. How does water interact with formic acid and methylene in reactions?
Water often acts as a solvent and nucleophile in reactions involving formic acid and methylene, especially in ester hydrolysis.
For More Posts And Updates: Vintage Posts

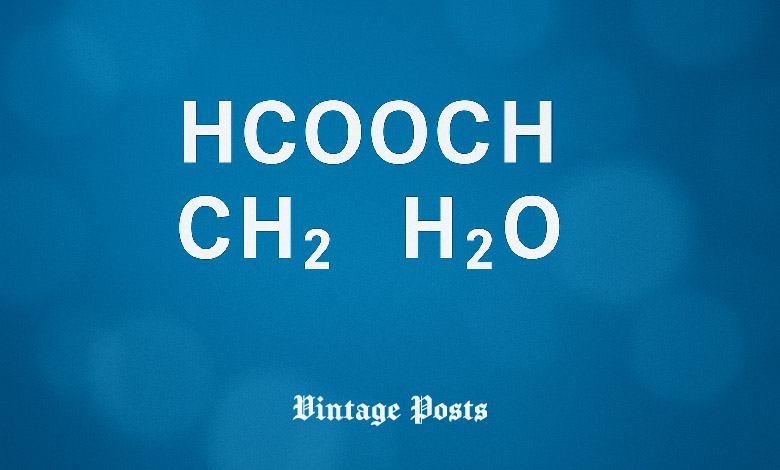













Leave a Reply